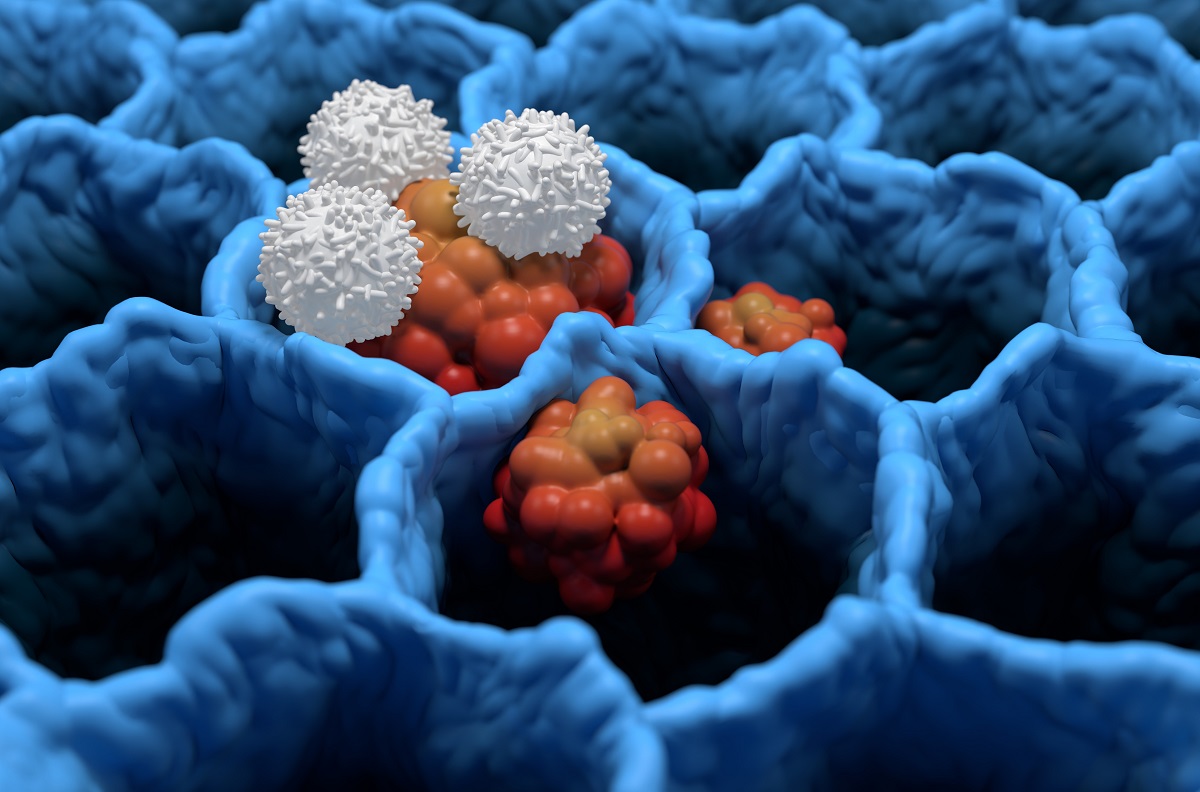
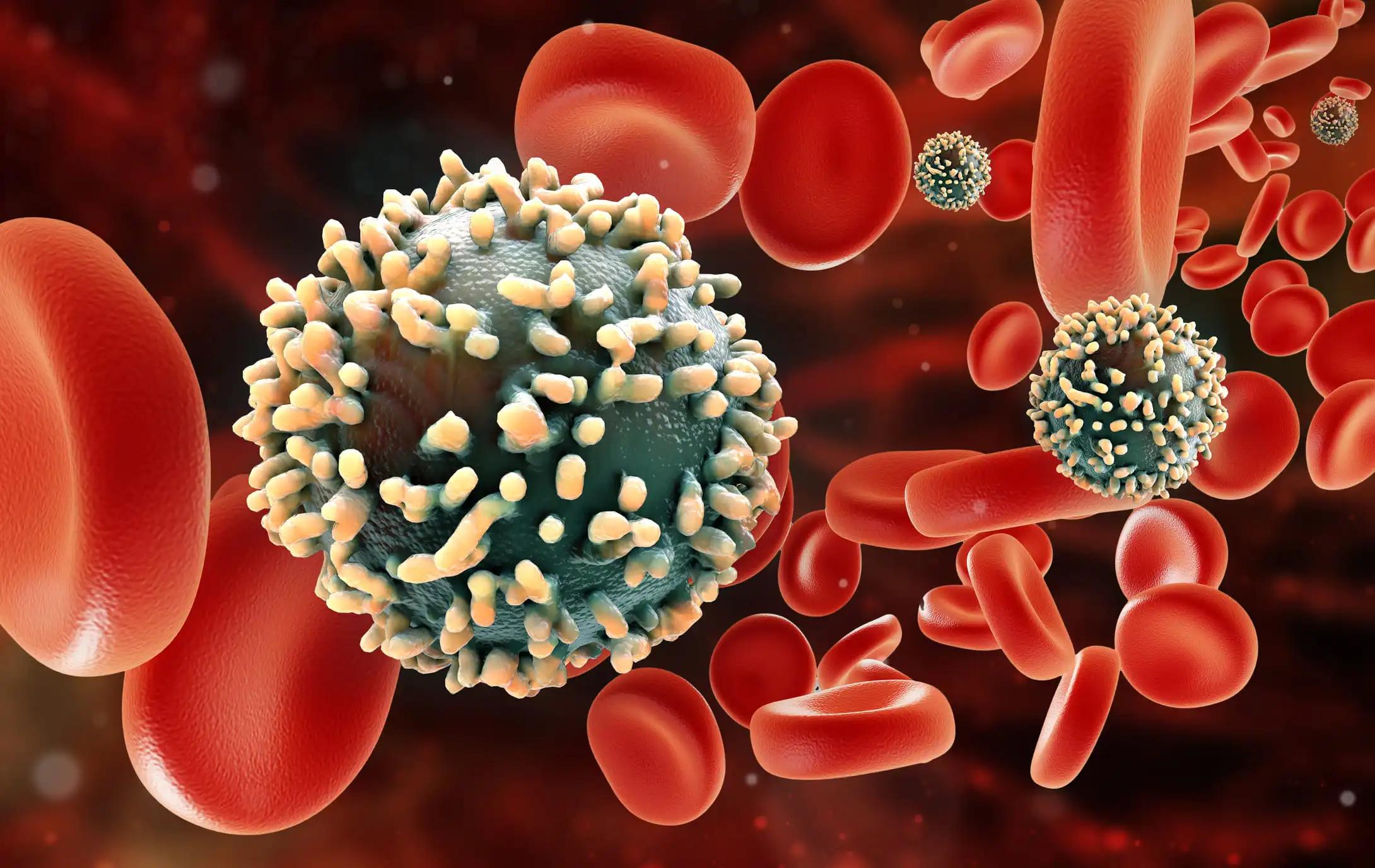
FDA has cleared Vironexis Biotherapeutics Investigational New Drug (IND) application for VNX-101, for the treatment of CD19+ acute lymphoblastic leukemia (ALL).
In Q4 2024, Vironexis anticipated launching a phase 1/2 trial for VNX-101, the first AAV-delivered cancer immunotherapy.
The trial offered hope for CD19+ ALL patients as VNX-101 had received FTD and Rare Pediatric Disease Designations from the FDA.
Samit Varma, CEO of Vironexis, expressed excitement about the drug clearance and highlighted the advancements in AAV-delivered T-cell immunotherapy -VNX-101.
He highlighted the potential of Vironexis to greatly improve the safety, effectiveness, and longevity of CD19+ ALL treatments, while also noting the rapid development of their pipeline and lead program within just three years, transforming the future of cancer care.
Dr. Cripe, Co-Founder of Vironexis, highlighted AAV’s proven efficacy and potential to overcome challenges of first-generation T-cell therapies like CAR-T.
He expressed excitement about moving this technology into clinical settings to broaden the benefits of VNX-101 for CD19+ ALL patients.
Steve Jurvetson, Co-Founder of Future Ventures, noted that the TransJoin technology’s broad applicability for treating blood cancers, preventing solid tumor metastasis, and developing cancer vaccines is exceptional.
Source: Vironexis Biotherapeutics Launches with FDA Clearance of IND Application for First-Ever Clinical Trial of an AAV-delivered Cancer Immunotherapy
https://www.biospace.com/press-releases/vironexis-biotherapeutics-launches-with-fda-clearance-of-ind-application-for-first-ever-clinical-trial-of-an-aav-delivered-cancer-immunotherapy